Badino G.
Abstract: This paper considers the different processes that can create vapour pressure above the equilibrium in the cave atmosphere: ascending air parcels, pressure drop behind bottlenecks, mixing of saturated air parcels at different temperatures and water flow fragmentation. These processes are essentially the same as those leading to clouds forming in the open atmosphere, always connected with air movements.
The difference of adiabatic lapse rates of water and moist air creates temperature imbalance between the flowing fluids in deep underground systems, leading to thermal and water exchanges, in which water flow globally subtracts energy from the system. The high purity of caves atmospheres tends to delay condensation. Condensation is concentrated where airflows are in close contact to the cave wall. The rate of aggressive water condensation on the walls is comparable to the external rain and can play a leading role in Speleogenesis.
Keywords: cave atmosphere, condensation in caves
Introduction
The water vapour pressure in the air above a flat surface increases until the number of molecules that evaporate from surface becomes equal to the number of those that come back. Thenceforth the evaporation still goes on, but the vapour partial pressure remains stable and equal to the equilibrium pressure, often incorrectly called “saturation” pressure (hereafter we shall use “saturated” air parcel, for simplicity - but some attention should be payed to the inaccuracy of using the word “saturation” in place of “equilibrium” - see Bohren, 1998). The value of the vapour equilibrium pressure depends essentially on the temperature of the system (water and vapour, which must be the same because thermo-dynamical equilibrium is assumed) as described by the Clapeyron equation. Secondly, it depends on the radius of curvature of free water surfaces (Kelvin equation).
The humidity in cave atmospheres is generally at the equilibrium level, due to the close contact among water surfaces and air in these quasi-closed systems, which allows them to reach equilibrium.
It is nevertheless very interesting to understand what happens when the vapour pressure is below or above the equilibrium level (from now on: sub- and super-saturated), because in these cases evaporation and condensation occur. Direct measurements of fluctuation amplitudes around the equilibrium value are unfortunately impossible because the usual hygrometers and evaporimeters do not reach such a high accuracy.
Nevertheless, direct observations show that water vapour is very often super-saturated. It is quite common to see cave walls covered by a film of water , to find droplets on the surfaces of objects left underground for some time, to see some haze in large halls and aerosols in the air: all these phenomena are possible if and only if the water vapour pressure is above equilibrium.
There is also indirect evidence of condensation processes at work, for instance, the so called “degree rule” (air warmer than water by some tenths of degree, a fact that by itself would break not less than the Second Principle...); the uniform rock surface dissolution, that extracts insoluble components from the rock (a corrosion obviously due to condensation in vadose conditions); other phenomena that can only result by moist air transformation.
The speleogenetic role of condensation-evaporation processes has been hypothesized since a long time. Trombe (1952) assumed that condensation processes on cave walls were driven by the thermal imbalances between air and water (the “degree rule” of Trombe, 1952). On the contrary, in my opinion the imbalance is coming from condensation, but that does not matter. What appears evident is that complex transformations take place among moist air and its constraints (the walls), so clearly that cavers often judge caves to be “active” or “fossil” depending upon the perception of water films on the walls.
The main aim of this work is to emphasize that super-saturation, and as a consequence condensation on walls, thermal disequilibria and so on are due to air movements, exactly as what happens in the outside atmosphere and with essentially the same processes.
In absence of motion, the system tends quickly to equilibrium (constant temperature and vapour pressure) where thermal and vapour exchanges cease.
The condensed water can have an important speleogenetic role because it is in equilibrium with local carbon dioxide and it is completely unsaturated for what regards salts.
In fact, these transformations are energetically fed by the air flux. It will be shown here (as already noted in Badino, 1995) that water subtracts energy from the mountain: when caves are excavated by condensation corrosion, the water acts as the chisel, but the hammer is the airflow.
It is important to note that these condensation processes in deep karst are not responsible for the net flux at the springs, which is in general very small (Dubliansky, 2000). In fact, estimations of water vapour density and average air flux through the deep karsts (maxima of 10 m3 s-1 km-2) give very small net amounts, which can be significant only for air fluxes through big stone chokes (Badino, 2004). Here we do not deal with the net condensation flux into a mountain, but with the condensation-evaporation fluxes due to inner cycles driven by air movements.
It is now useful to estimate the speleogenetic capability of water flowing inside a cave, in order to compare it with the following estimations of water condensation. The annual precipitation level in the Alps is of the order of 1000 mm, which corresponds to about 30 mg s-1 m-2. Only a part of this infiltrates underground and becomes quickly enriched with carbon dioxide from the soil and with carbonate salt from contact with the rock, with complex kinetics (Dreybrodt, 2000). Therefore, the residual dissolving capability of water at depth is a very small fraction of the precipitation and depends on the details of the absorption circuit and we can estimate that it is equivalent to an unsaturated water flux of 0.1-10 mg s-1 per square metre of external surface. These figures will be compared to the estimated water depositions from “underground clouds”.
The temperature gradient in deep karst
Condensation and evaporation processes in caves are due to air movements into the mountain that force the gas to continuous transformations. It is very important to discuss the global thermo-dynamical conditions of cave atmospheres, starting from their main term, the temperature variation with altitude (temperature gradient).
The vertical gradient in the Earth free atmosphere near the surface is, on average, -6.5 °C km-1 (Bohren, 1998). In fact, if a perfectly dry air parcel is considered, then it is easy to see that in an ideal atmosphere (in hydrostatically neutral equilibrium) the temperature gradient would be -9.7 °C km-1, the so-called “dry adiabatic lapse rate”. This is no longer true when the air parcel contains water vapour: in fact, when the cooling causes a supersaturation, some vapour condenses, releasing its vaporization enthalpy, and thus reducing the temperature decrease.
The absolute value of the lapse rate becomes then smaller, but the exact value now depends on the water vapour content, which in turn depends on temperature and pressure (“moist adiabatic lapse rates”). Table shows some example values in usual karst conditions.
TABLE:
Moist adiabatic lapse rate (-°C/km) vs. altitude and temperature
|
Altitude (m) |
100 |
1000 |
2000 |
3000 |
4000 |
|
T=0°C |
6.58 |
6.38 |
6.14 |
5.91 |
5.70 |
|
5°C |
5.90 |
5.70 |
5.50 |
5.25 |
|
|
10°C |
5.32 |
5.11 |
4.89 |
4.68 |
|
|
15°C |
4.80 |
4.60 |
4.40 |
4.20 |
|
|
20°C |
4.35 |
4.17 |
3.98 |
3.80 |
|
|
25°C |
3.95 |
3.80 |
3.65 |
|
|
|
30°C |
3.63 |
3.49 |
3.35 |
|
|
It is then reasonable that the atmospheric average lapse rate be near the moist adiabatic one, because of the air humidity contents.
Surprisingly, the obvious conclusion that the same value can be found in caves, where relative humidity is usually 100%, is not true, and the underground lapse rate is significantly different; this comes from the fact that the cave temperature is mainly established by the water flowing into it, given that, as a rule, the total thermal capacity that enters underground is mainly in the water flux.
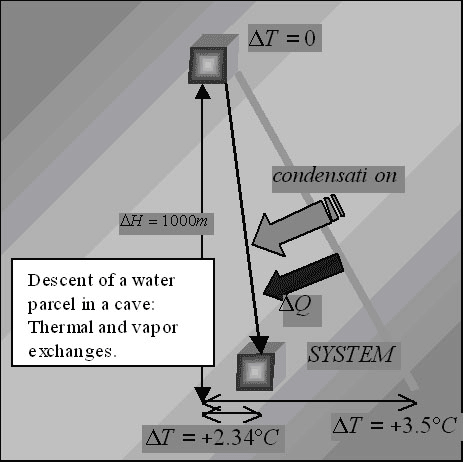
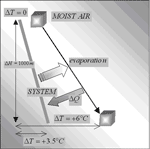
Fig. 1. Thermal and vapor exchanges of a descending water parcel underground: its adiabatic lapse rate is –2.34 °C/km, in absolute value smaller that the typical caves lapse rates, which means that generally water flow subtracts energy from deep karst.
It is easy to show that the complete transformation of an initial gravitational potential energy into internal energy (adiabatic assumption) heats a water parcel of 2.34 °C per kilometre of fall (“water adiabatic lapse rate”). This would in fact be the asymptotic lapse rate in a cave dominated by water fluxes.
A large set of measurements has shown that the real situation is intermediate, the cave lapse rates lay between the water and moist air ones (Badino, 2000). In deep alpine caves the lapse rate stays between -2.8 and -4 °C km-1. Only in very special cases, for instance the quartzite shafts in the Tepuis of Venezuela, it is possible to measure lapse rates that are close to the moist adiabatic one.
The “water adiabatic lapse rate” is of paramount importance, because it is completely different from the moist air one. The geothermal energy flux cannot play any role in this temperature increase along the cave, because it is completely intercepted by the deep water drainage structures. It has already been emphasized (Badino, 1990) that this temperature excess above the adiabatic water transfer has a simple but huge implication: the water flows out from the deep karst warmer than in the adiabatic model, which means that it subtracts energy from the mountain (Fig. 1).
Clouds from the ascent of air parcels
The ascent of a saturated air particle is the main process able to create supersaturation conditions in the air parcels, leading to cloud formation in the free atmosphere and, for example, to higher precipitation for mountains that are able to force air flows upwards.
The rise of saturated air particles plays a similar role underground: the rising air parcels tend to condense on the walls, while the descending particles tend to evaporate. It is possible to discuss these processes in more detail.
The deep karst temperature gradient is intermediate between the water and moist air adiabatic lapse rates. This implies that each one of the two fluids in the vertical transfers into the mountain undergoes transformations that are not adiabatic. There is a continuous thermal exchange between the two and the transformation can be considered “with constant temperature gradient” (Badino, 1995). A detailed analysis of this transformation, which can be reduced to a particular politropic, is beyond the scope of this paper, but one example can be given.
Consider for instance the ascent of an air parcel (one cubic meter) with vapour at the equilibrium point and take as an example a rise of 1 km in a cave at 10 °C with an internal lapse rate of –3.5 °C km-1. From the energetic point of view, the transformation can be split into two steps: an adiabatic ascent to the final altitude and an isobaric transformation to the local conditions (Fig. 2).
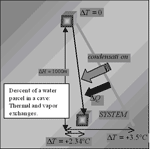
Fig. 2. Thermal and vapour exchanges of an ascending air parcel underground: as it happens in the free atmosphere, air ascent creates supersaturation and cloud formation.
The adiabatic ascent is “moist adiabatic”, giving a total cooling of –5°C and condenses roughly 5 g of water vapour. The subsequent heating to the local conditions requires an enthalpy increase to heat the air parcel of 1.5 °C and to also vaporize of 1.5 g of water. This shows that in the whole process two processes took place:
1) The air parcel absorbed thermal energy from the internal surroundings.
2) It released 3.5 g of water per cubic metre.
In the real case the energy and water exchanges are distributed along the path (see more details below). Generally, the ascent of an air parcel takes place during the cold season, but we should note that this mechanism also works in the case of an ascending branch of a globally descending flux. This means that the underground “rainy season” is in general winter, but locally it can be summer...
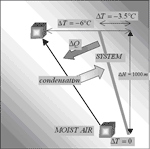
The descending air parcel obviously tends to evaporate water and to dry the cave (Fig. 3), probably with a tendency to create thermal imbalance in the region already dried. For instance, if there is not much water to evaporate on the conduit ceiling, the flowing air parcel tends to warm more and to stay “trapped” in the upper parts of galleries: thermal sedimentation can then occur along the flow.
Fig. 3. Thermal and vapour exchanges of a descending air parcel underground: the moist air adiabatic lapse rates are larger than that of water and those of caves; the air transfers are then able to create thermal disequilibria.
It is quite easy to estimate the total water release: e.g. in alpine karst it is quite usual to have a flow of 10 m3 s-1 along the main stream on vertical transfers of about 1 km. This flow is able to release scores of grams on the walls. Assuming active surfaces of some 104 m2, we have water deposition of the order of 1 mg m-2 s-1.
We shall see below that these deposits are in fact concentrated where the conduits are smaller.
Underground banner clouds
Banner clouds are those clouds that sometimes appear leeward of large peaks. Their phenomenology is not completely understood, but they appear to be the result of a sudden (i.e. adiabatic) air expansion that locally cools the gas below the dew point (Friedlander, 2000).
Let us now consider the airflow in a cave system. The flux through a mountain, as a whole, can be considered a Joule-Thomson expansion (note that it is locally called “expansion” either for ascent or descent of air parcels) because in fact the air particle is “throttled” through the cave. The general transformation is then isenthalpic, as the flow through a single narrow passage.
A much more accurate discussion would be necessary because we should discuss constant, periodic or transient flows, the role of rock surfaces and so on, but approximately we can say that downstream the air parcel undergoes a sudden expansion that locally (in time and space) can be considered adiabatic and therefore resulting in a temperature drop.
The pressure drop, roughly proportional to the square of velocity in the “throttle”, becomes quite small as the temperature decreases. We can use, for comparison, the vertical pressure drops and the corresponding temperature drops for ascending air parcels (moist adiabatic).
For example, a velocity of 5 m s-1 corresponds to a pressure decrease of about 10 Pa, equivalent to an ascent of one metre with a moist adiabatic transformation and then to a temperature drop of 5 mK. The supersaturation level would then be about 0.05% of the relative humidity, which is not directly measurable but is enough to create condensation on the walls; the estimation is then 5 mg per flowing cubic metre, concentrated near the narrow passage.
As in the case of external banner clouds, the super-saturation disappears downstream, re-evaporating the aerosol that can anyway be effective in local rock dissolution just near the throttle.
Mixing clouds
The mixing clouds in caves are so common that they are almost not perceived: the clouds that cavers create with breath or moist clothing are typical mixing clouds. In literature (Bohren, 1998), it is possible to find discussions about the mixing clouds formation mechanism and its common misinterpretation, that is believing they form “because the warm breath is cooled...” and so on: “Heating and cooling per se are irrelevant... mixing clouds are formed by mixing of different air parcels... because of the shape of the saturation vapour pressure curve two parcels can mix to form a supersaturated parcel” (Bohren, 1998). More recently their role in caves atmosphere was considered (Lismonde, 2002).
This is in fact a mechanism connected with the non-linearity of the Clapeyron curve (Fig. 4), and essentially the same as the so-called “Bögli’s mixing corrosion”.
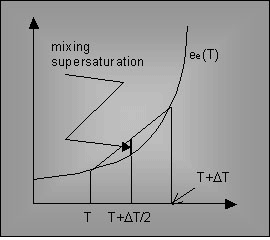
Fig. 4. The Clapeyron curve and the result of mixing of two air parcels: the mixed air parcel is always supersaturated due to the curve concavity.
Temperature drops in caves are quite common due to different “histories” of water and air columns flowing along different branches, often characterized by different temperature gradients along the flow but mainly by different temperature at the entrance (Badino, 1995).
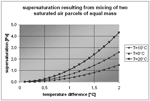
Temperature drops of 1°C, or more among different branches are common in alpine karst, especially in presence of very steep external topographies. Calling T and T+ΔT the absolute temperature of the two saturated air parcels, with equal mass, ρv the equilibrium vapour density (absolute humidity) at T, it is quite easy to calculate the supersaturation by Clapeyron equation. It is possible to show (Appendix A) that the equation giving the relative quantity of vapour in excess as compared to its equilibrium value, as function of their temperature difference, it is given by
It is easy to see that these super-saturations are low and not directly measurable, some 0.01-0.1% of relative humidity, which nevertheless correspond to a total release of some milligrams per cubic metre of air coming at the mixing point. The conduit confluences are then preferred regions for water deposition on the walls.
Fig. 5. Relative quantity of vapour that condenses after the mixing of two air parcels of equal mass, as function of their temperature difference.
Fragmentation clouds
Up to now three internal phenomena have been described, the microscopic side of well-known macroscopic events, that correspond to large-scale external effects.
Fragmentation clouds are quite different. The processes that generate clouds from fragmentation of cascades obviously do exist outside, but their role in the open atmosphere is quite negligible. In the case of closed or semi-closed water draining systems, like caves or canyons, this is no longer true.
The main point is that the Clapeyron law describes the equilibrium pressure above a flat water surface. If the surface has a radius of curvature comparable with the intra-molecular interaction lengths, significant corrections have to be done. In practice, a molecule on the surface of a small droplet is less bent to the liquid than in a flat surface and its tendency to evaporate is stronger. The equilibrium pressure is then higher (Fletcher, 1969; Rogers, 1989).
The equation connecting the es (equilibrium pressure) on a flat surface and the esr on a droplet is given by the Kelvin equation.
Fig. 6 shows the supersaturation level on a flat surface having a moist air in equilibrium with droplets of radius r.
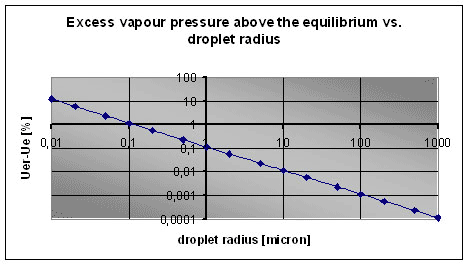
Fig. 6. Supersaturation above droplets of radius r in comparison with a flat water surface.
Incidentally we then note that the very common presence of a stable aerosol (or haze) in a cave atmosphere is a direct evidence of a supersaturation of moist air.
Let us try to estimate the typical supersaturation levels, drawing a distinction among the droplets created by free fall and those created by impact.
A droplet falling in the air moves at a constant velocity, established by the equilibrium between viscous and gravitational forces. If the droplet is sufficiently small it moves in a laminar regime, but if its radius is larger than a critical value of 0.5 mm, the Reynolds number associated with its motion becomes higher than 2000 and the regime becomes turbulent. The dissipated power increases and lee eddies appear, transmitting oscillations to the droplet. In practice, a large drop tends to become unstable and to subdivide into droplets of size smaller than the critical one. It would be very important to perform measures, but in the meanwhile we can assume that the fragmentation during fall creates droplets, mainly with a quite large size (hundreds of micron) and then with small associated supersaturation.
The final fragmentation on rock or water is more efficient for splitting droplets because deceleration is higher than in the case of falling droplets; if we assume that the final kinetic energy is mostly spent in creating droplet surfaces, we obtain a typical size of 10 µm.
Fragmentation is then able to mechanically create a cloud, which has to be in equilibrium with the surrounding air. The surrounding rock surfaces are then washed not only by the falling drops and aerosol, but also by the condensation of supersaturated air, due to fragmentation and to vertical eddies moved by the waterfalls.
It is easy to see that also in this case the mechanism produces supersaturation of some 0.01%, against an excess water content of milligrams per cubic metre, that are going to be released on the walls when the air parcel eventually flows on it.
The Kelvin equation is useful also for a negative radius of curvature, that is for a concave surface. In this case the surface needs a smaller vapour pressure to reach equilibrium and then it becomes a preferred condensing point for surrounding air in equilibrium with a flat surface. This means that small fissures tend to fill with aggressive condensed water.
Condensation nuclei
It is well-known that super-saturated air particles can only condense in presence of “condensation nuclei”, that is, air impurities. Theoretically, condensation cannot begin in absence of nuclei because it would start with very small droplets immediately evaporating due to the high supersaturation level required for their stability. It is possible to show that condensation also occurs in absence of nuclei (homogeneous nucleation), because some molecular clusters are always present in air (Friedlander, 2000); in this case, however, really high supersaturation values are required, probably impossible to be attained underground.
This explains why the cave atmospheres are so pure: the supersaturated water vapour (ascents, eddies, mixing, expansions...) is able to capture and precipitate dust from the air to the floor, thus removing particles. Once the purification process is completed, condensation on nuclei in the air flow becomes impossible and significant supersaturation can occur. Because rock surfaces are excellent condensation “nuclei”, a locally supersaturated air parcel will condense (performing a “supersaturation reset”) as soon as it comes in contact with rocks.
It is easy to understand that the points where supersaturation is more efficiently reduced are those where the conduit sizes are smaller, either for the favourable surface-volume ratio or the stronger mixing due to the air flow acceleration. The smallest cave parts along the air column flow are then preferred points for condensation. In these points the supersaturations formed in large regions upstream are integrated, and this fact has obvious consequences on speleogenesis: the conduit sizes along the airflow tend to be constant, aside from the calcium carbonate saturation of local water flows.
Conclusions
This discussion has outlined general processes, without trying to discuss details that would turn this short note into a book. This paper is a small part of a larger work on general underground climate physics currently being prepared following the introduction given in Badino (1995).
Nevertheless many points are here too poorly discussed. The most important is probably the energy balance and kinetics of supersaturation formation, that leads to thermal imbalances between the aerosols and the air. Other important points have been ignored, like the kinetics of droplet evaporation and formation, thermal sedimentation in air, the presence of dissolved salt in fragmented water, the dynamics of air flow on walls and the dynamic of aerosol size-spectra.
These problems have already been partly analyzed, but unfortunately present extreme calculation complexities and progress has been inhibited by a complete lack of measurements. This brief, semi-qualitative paper has been written in order to suggest further observations, promote discussion and additional theoretical studies.
References
- Badino G. 1995. Fisica del Clima Sotterraneo. Memorie dell’Ist. Italiano di Speleologia, 7, serie II, Bologna
- Badino G. 2000. I Gradienti di Temperatura nei Monti, un Indicatore Esplorativo, Talp-FST, 21, 72-80
- Badino G. 1990. Microclima ed Energetica. In: “Il Complesso Carsico di Piaggia Bella”, AGSP, Reg. Piemonte, 47-59
- Bohren C. and Albreht B. 1998. Atmospheric Thermodynamics. Oxford Univ. Press, 1998, 402 pp.
- Dreybrodt W. 2000. Equilibrium Chemistry of Karst Waters in Limestone Terranes. In: Klimchouk, A., Ford, D.C., Palmer, A.N., and Dreybrodt, W., (Eds.), Speleogenesis: Evolution of karst aquifers. Nat. Speleol. Soc., USA, 126-135.
- Dublyansky V and Dublyansky Yu. 2000. The Role of Condensation in Karst Hydrogeology and Speleogenesis. In: Klimchouk, A., Ford, D.C., Palmer, A.N., and Dreybrodt, W., (Eds.), Speleogenesis: Evolution of karst aquifers. Nat. Speleol. Soc., USA, 100-112.
- Fletcher N. H. 1969. The Physics of Rainclouds. Cambridge Univ. Press, 389 pp.
- Friedlander S. 2000. Smoke, Dust, and Haze. Oxford Univ. Press, 408 pp.
- Lismonde B. 2002. Aérologie des systèmes karstiques, vol II, CDS Isére, 362 pp.
- Rogers R. ET AL. 1989. A Short Course in Cloud Physics. Pergamon Press.
- Trombe F. (1952. Traité de Spéléologie. Payot, 1952, 376 pp.